Shop
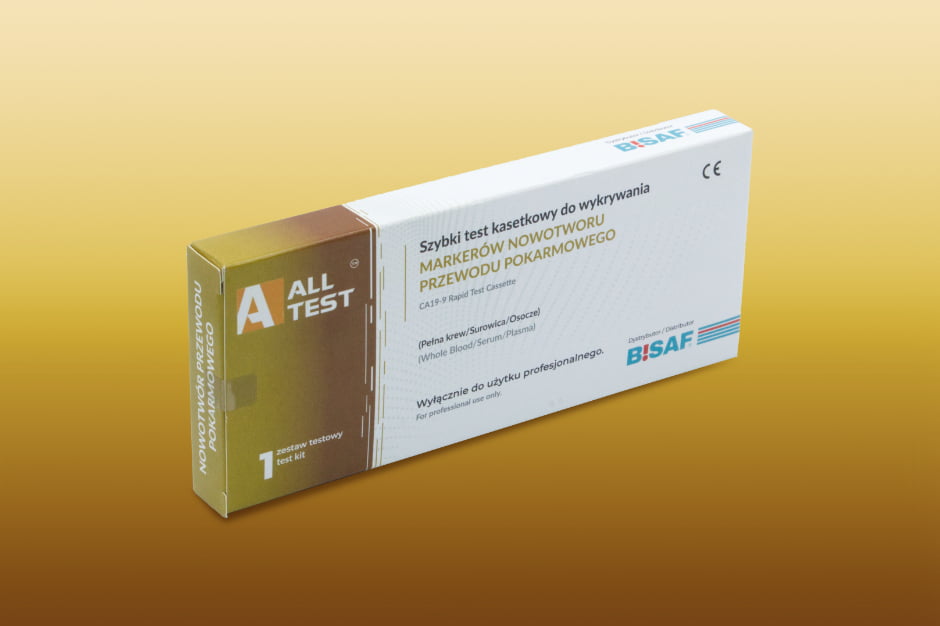
CA19-9 test | gastrointestinal cancer markers | 1 piece
24,99 PLN per piece
Work efficiently, order profitably!
Place wholesale orders at info@bisaf.pl
Product designed for professional use.
In stock
Product description
Test for CA19-9 antibodies to gastrointestinal cancer
The CA19-9 rapid cassette test is a rapid, chromatographic immunoassay for the qualitative detection of CA19-9 antibodies to gastrointestinal cancer in a sample of whole blood, serum or plasma. CA 19-9 tumor antigen is a protein present in the body whose levels can increase in certain types of cancer, mainly pancreatic cancer, colorectal cancer and gastric cancer. It is used as a diagnostic marker and to monitor the course of treatment for these diseases, allowing assessment of response to therapy and possible recurrence.
The high sensitivity and specificity of this test allow for reliable and accurate results, which are obtained in as little as 10 minutes!
The product is intended for professional use.
This is a medical device. Use it in accordance with the instructions for use or the label.
Properties
- sample material: whole blood, serum or plasma
- convenient collection of the test sample
- specificity: 98.7%, sensitivity: 96.9%, accuracy: 98.3%
- high quality product
- quick result in as little as 10 minutes!
- single-packaging
- product designed for professional use
The package contains
- test cassette
- pipette
- buffer
- alcohol swab
- lancet
- instruction manual
Test Applications
Our test is ideal for professionals in various fields, especially gastroenterologists, internists and oncologists, for whom it can be helpful in:
- diagnosing early stage cancer: CA19-9 tests can be used as part of the diagnosis of early-stage pancreatic cancer and colorectal cancer, enabling faster identification of the disease and increasing the chances of successful treatment
- monitoring the effectiveness of therapy: CA19-9 tests allow doctors to monitor a patient’s response to cancer treatment. A decrease in the level of the antigen may suggest a positive response to therapy, while an increase may indicate a potential recurrence or resistance to treatment
- detecting relapse: after cancer treatment, there is a risk of recurrence. Regular testing of CA19-9 levels can help detect recurrence early so that therapeutic action can be taken quickly
- health care planning: CA19-9 tests can be used in determining the health care plan for patients with pancreatic or colorectal cancer. The test results help doctors adjust therapy and monitor disease progression
- screening: in some cases, CA19-9 tests can be used as part of screening for patients at increased risk of cancer, such as those with a family history of the disease or other risk factors
Test Procedure
Note: to make the test performed as reliable as possible, the test should be stored in a dry place at 2-30°C and used before the expiration date. Do not drink, eat or smoke in the area where the sample is stored and the test is performed, as this may affect the result obtained. Make sure to bring the sample, cassette and buffer to room temperature (15-30°C) before performing the test.
- Remove the test cassette from the sealed package.
- Clean the patient’s finger with a disinfecting swab.
- Massage the hand without touching the puncture site, rubbing it towards the tip of the selected finger (preferably middle or ring finger).
- Puncture the skin with a sterile lancet. Wipe off the first traces of blood.
- Gently rub your hand from wrist to finger to form a rounded drop of blood on the surface of the puncture site.
- Collect blood from the patient’s finger using a capillary, avoiding air bubbles.
- Drop 3 drops of sample (about 75 μL) onto the cassette, then add 1 drop of buffer (about 40 μL) and start timing.
- Read the result after 10 minutes.
Product Identification Data and Responsible Entities
EAN Code: (01)69369831359316
| Manufacturer | Authorized Representative | Importer / Distributor |
| Hangzhou AllTest Biotech Co., Ltd 550, Yinhai Street, Hangzhou Economic & Technological Development Area 310018 Hangzhou China css21@alltests.com.cn +86 57156267147 www.alltests.com.cn |
MedNet EC-Rep GmbH Borkstrasse 10 48163 Muenster Germany birthe.harms@mednet-ecrep.com +49 2513226661 www.mednet-ecrep.com |
Bisaf Sp. z o.o. Rdestowa 5 54-530 Wrocław Polska 24h@bisaf.pl +48 503411527 www.bisaf.pl |
Bestsellery
432,00 PLN per package (25 pieces)
Last watched
432,00 PLN per package (25 pieces)