486,00 PLN per package (25 pieces)
Shop
Fluorecare COMBO 9in1 test | SARS-CoV-2 | Influenza A+B | RSV | Adenovirus | hMPV | Rhinovirus | PIV | Mycoplasma Pneumoniae | 1 piece
59,99 PLN per piece
Work efficiently, order profitably!
Place wholesale orders at info@bisaf.pl
Intended for professional use.
Skontaktuj się z nami / Contact us
Product description
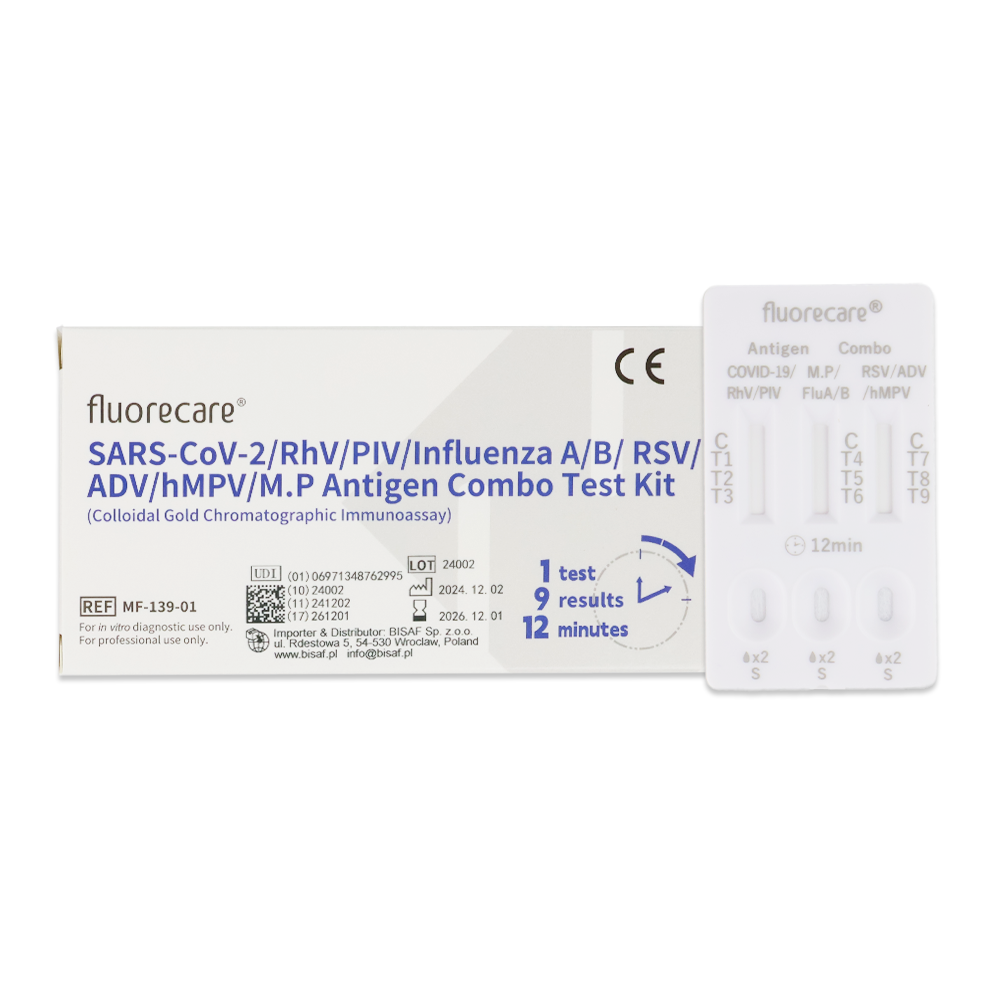
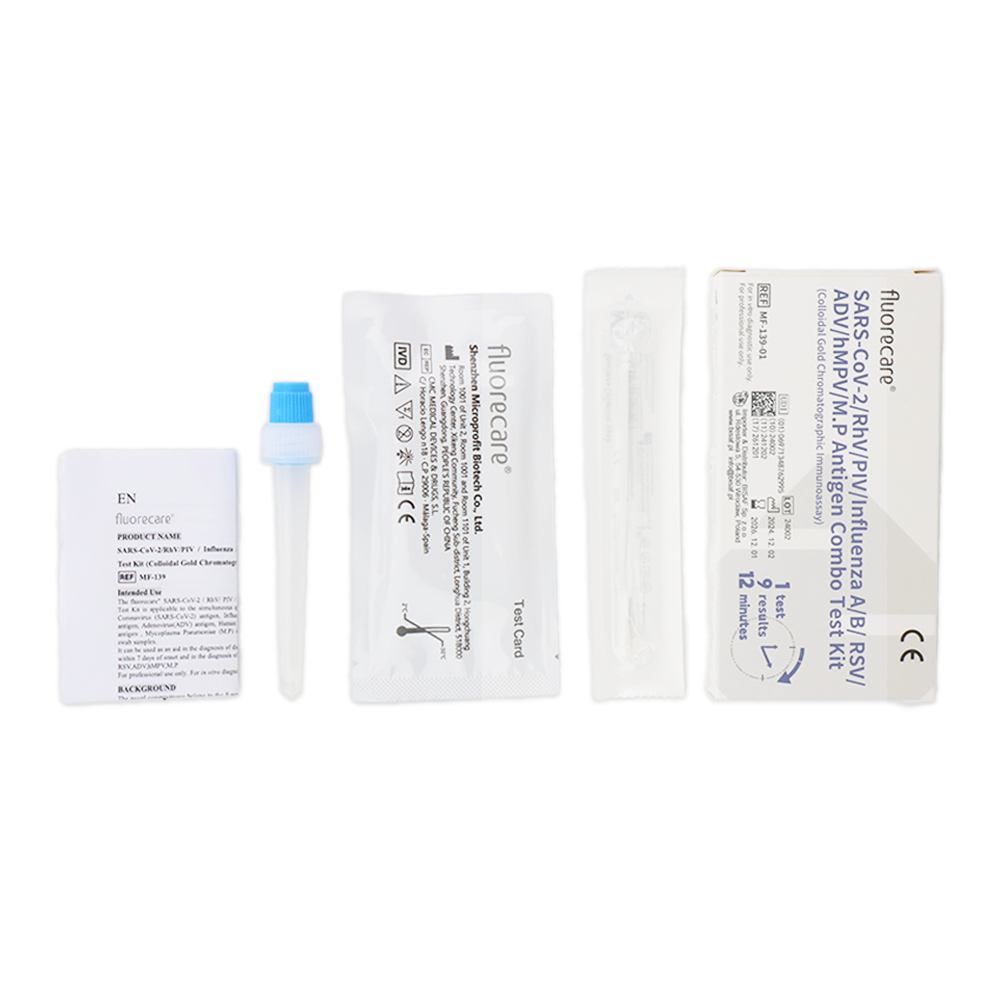
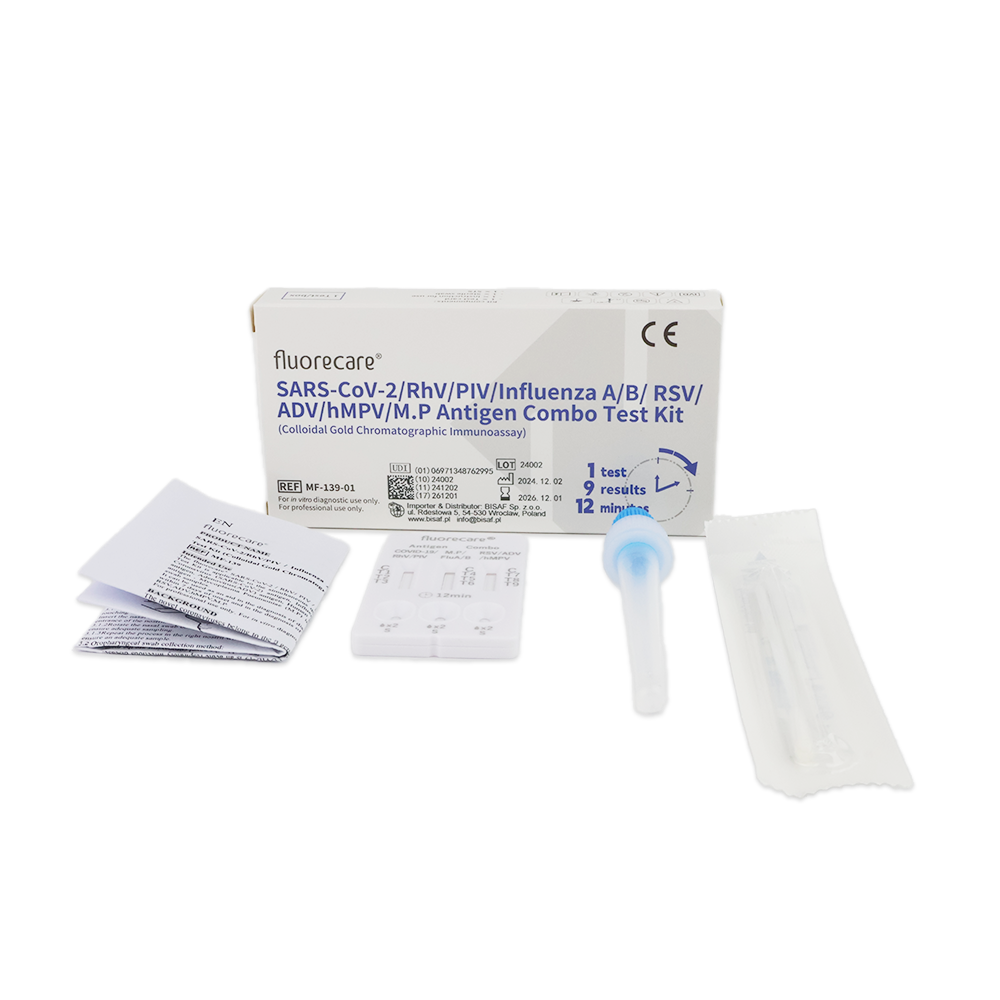
Fluorecare COMBO 9in1 test (nasal swab/nasopharyngeal swab/throat swab)
This is a rapid test designed for the qualitative and differential detection of antigens for SARS-CoV-2, Influenza A and B, RSV, Adenovirus, Human Metapneumovirus (hMPV), Rhinovirus, Parainfluenza Virus (PIV), and Mycoplasma pneumoniae. The sample can be collected from the patient’s nose, nasopharynx, or throat.
1 swab = 9 results
The high sensitivity and specificity of this test ensure reliable and accurate results, available in just 12 minutes!
For professional use only.
This is a medical device. Use it in accordance with the instructions for use or the label.
Properties
- sample material: nasal swab, nasopharyngeal swab or throat swab
- painless sample collection
- high-quality product
- rapid results in just 12 minutes
- single packaging
- intended for professional use
Test specification
- SARS-CoV-2: specificity: 100%, sensitivity: 92.93%, accuracy: 96.11%
- Influenza A: specificity: 96.88%, sensitivity: 95.71%, accuracy: 96.27%
- Influenza B: specificity: 96.88%, sensitivity: 92.86%, accuracy: 94.78%
- RSV: specificity: 100%, sensitivity: 98.72%, accuracy: 99.44%
- Adenovirus: specificity: 100%, sensitivity: 98.06%, accuracy: 99.03%
- hMPV: specificity: 100%, sensitivity: 98.04%, accuracy: 99.05%
- Rhinovirus: specificity: 98.68%, sensitivity: 97.48%, accuracy: 98.06%
- PIV: specificity: 98.75%, sensitivity: 98.08%, accuracy: 98.42%
- Mycoplasma pneumoniae: specificity: 96.36%, sensitivity: 97.01%, accuracy: 96.72%
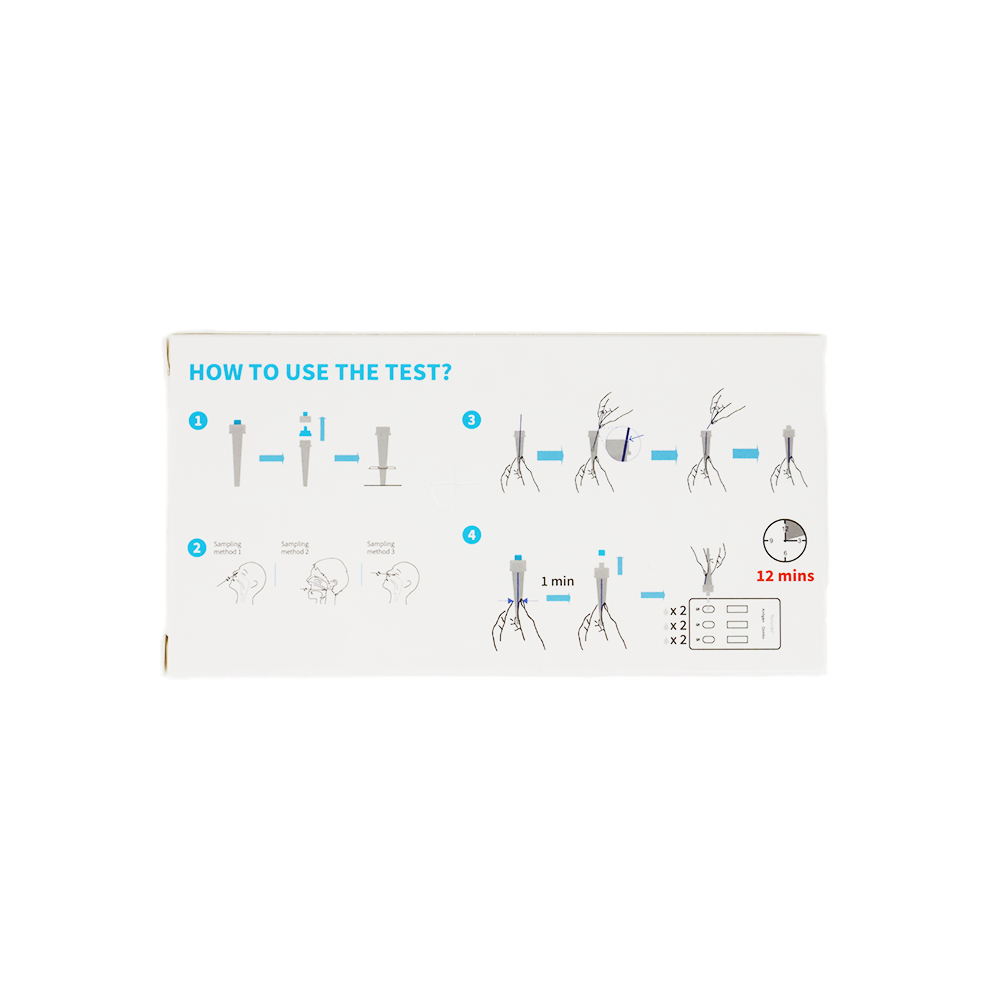
The package contains
- 1 test cassette
- 1 single-use sterile swab
- 1 extraction buffer
- user manual
Test application
- This test is designed for the qualitative assessment of infections caused by SARS-CoV-2, Influenza A, Influenza B, RSV, Adenovirus, Human Metapneumovirus (hMPV), Rhinovirus, Parainfluenza Virus (PIV), and Mycoplasma pneumoniae. It enables the rapid detection or exclusion of the cause of a patient’s symptoms.
- The test is particularly recommended for pediatricians, as children are exposed to such infections in school and preschool environments. Painless sample collection makes it suitable for the youngest patients.
- It is also intended for screening in primary care facilities and general practitioners’ offices, allowing for the rapid confirmation or exclusion of infection with one of the six most common respiratory pathogens.
Product Identification Data and Responsible Entities
EAN Code: 6971348762995
| Manufacturer | Authorized Representative | Importer / Distributor |
| Room 1001 of Unit 2, Room 1001 and Room 1101 of Unit 1, Building 2, Hongchuang Technology Center, Xikeng Community, Fucheng Sub-district, Longhua District, 518000 Shenzen China bio@microprofit.com +86 75529303416 www.microprofit-bio.com |
CMC Medical Devices @ Drugs, S.L. C/Horacio Lengo n18 C.P. 29006 Malaga Spain info@cmcmedicaldevices.com +34 951214054 www.cmcmedicaldevices.com |
Bisaf Sp. z o.o. Rdestowa 5 54-530 Wrocław Polska 24h@bisaf.pl +48 503411527 www.bisaf.pl |
Bestsellery
14,99 PLN per piece
Previous
Next
Last watched
297,00 PLN per package (25 pieces)
Previous
Next