Fluorecare COMBO 4in1 test | SARS-CoV-2 | Influenza A+B | RSV | 25 pieces
224,75 PLN per package (25 pieces)
8,99 PLN per piece
Work efficiently, order profitably!
Place wholesale orders at info@bisaf.pl
Product designed for professional use.
Availability: In stock
Product description
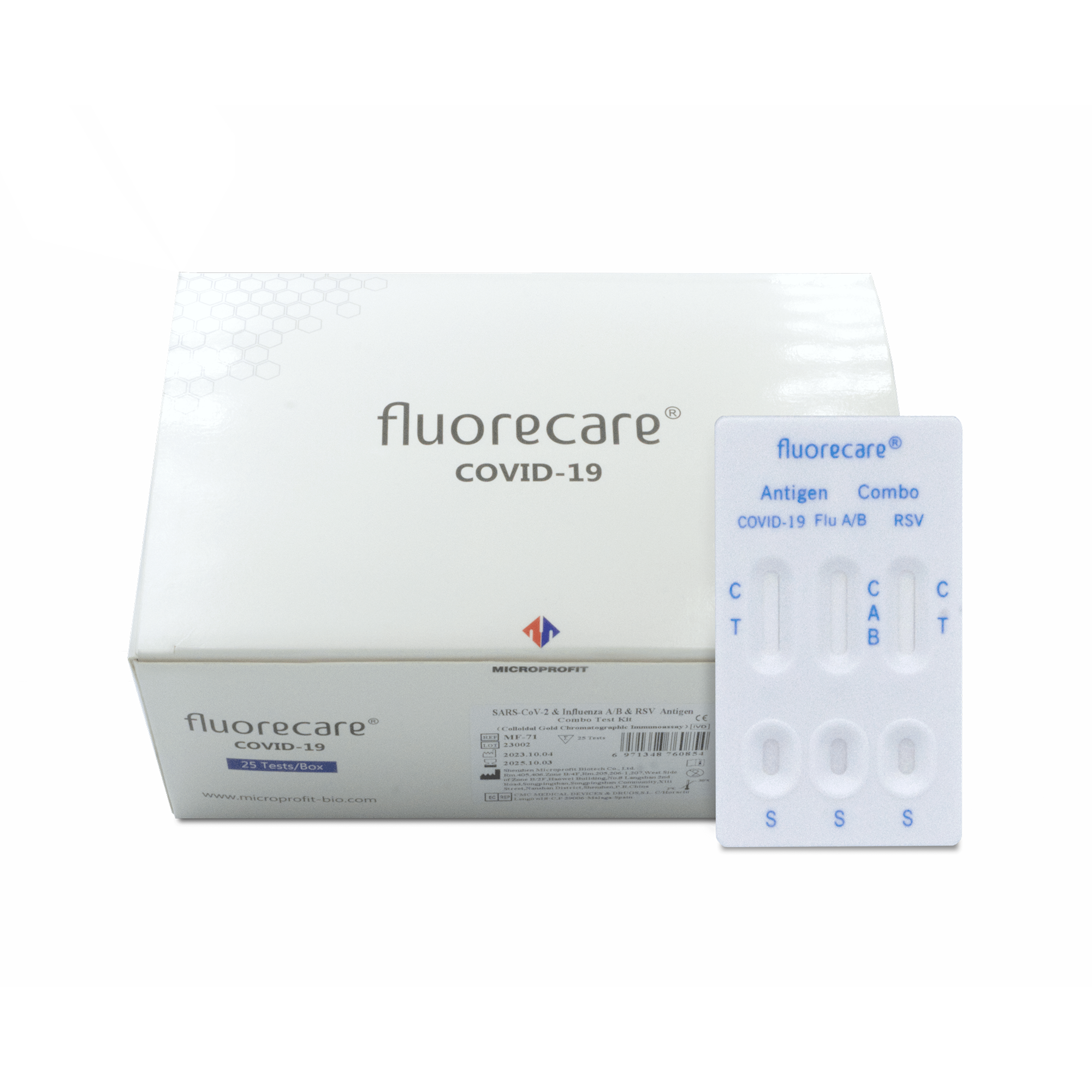
Fluorecare COMBO 4-in-1 Test Influenza A+B / COVID-19 / RSV
This is a rapid in vitro diagnostic test designed for the qualitative detection of SARS-CoV-2 antigens, Influenza type A (including subtype H1N1) and B antigens, as well as RSV virus using the colloidal gold method. It can be used as an aid in the diagnosis of infections caused by the mentioned viruses in individuals displaying characteristic symptoms or in asymptomatic individuals (in the presence of epidemiological reasons justifying suspicion of infection). The test can be performed using throat, nasal, or nasopharyngeal swab samples under in vitro conditions.
1 swab = 4 results
The high accuracy of this test allows for reliable results obtained in just 12 minutes!
Product intended for professional use.
This is a medical device. Use it in accordance with the instructions for use or the label.
Properties
- sample material: nasal swab, throat swab, or nasopharyngeal swab
- painless collection of the test sample
- high product quality
- quick result in as little as 12 minutes!
- bulk packaging of 25 units
- product designed for professional use
Test specification
- accuracy of SARS-CoV-2: 92.9%
- accuracy of influenza A: 91.8%
- accuracy of influenza B: 92.7%
- accuracy of RSV: 96.97%
The package contains
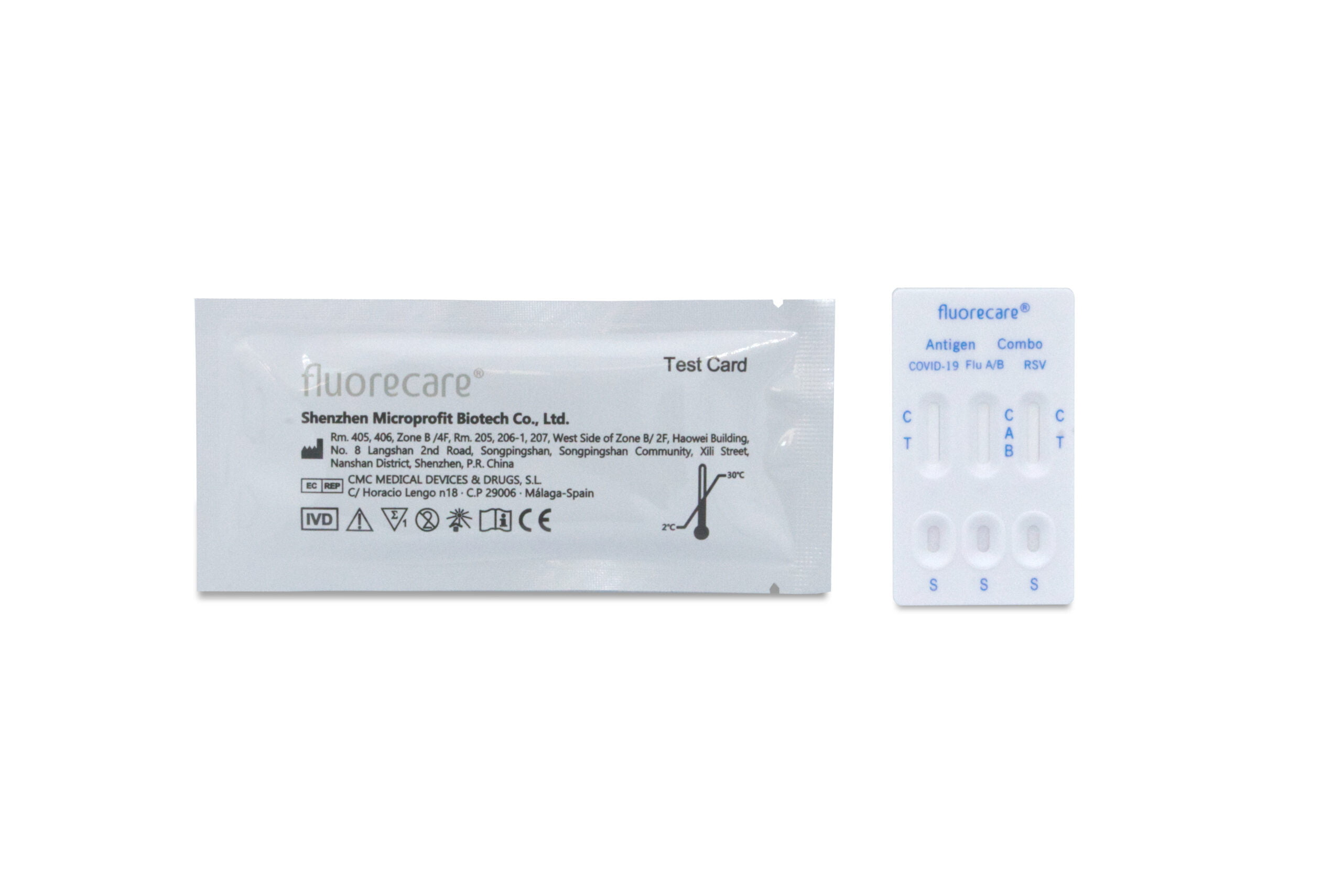
- 25 test cassettes
- 25 single-pack sample processing solutions
- 25 dropper cap covers
- 25 sterile swabs
- 25 extraction tubes
- instruction manual
Test Application
- this test kit is used for simultaneous qualitative detection and differentiation of the new Coronavirus (SARS-CoV-2 antigen), Influenza A virus, Influenza B virus antigen, and/or RSV antigen in population-based throat, nasal, and nasopharyngeal swab samples under in vitro conditions
- the test is intended for screening, making it an excellent tool for primary care physicians and general practitioners who can quickly confirm or rule out infections caused by one of the four most common respiratory viruses
Product Identification Data and Responsible Entities
EAN Code: 6971348760854
| Manufacturer | Authorized Representative | Importer / Distributor |
| Shenzen Microprofit Biotech Co., Ltd. Room 1001 of Unit 2, Room 1001 and Room 1101 of Unit 1, Building 2, Hongchuang Technology Center 518000 Shenzen, China bio@microprofit.com +86 75529303416 www.microprofit-bio.com |
CMC Medical Devices @ Drugs, S.L. Calle Horacio Lengo 18 29006 Málaga Spain info@cmcmedicaldevices.com +34 951214054 www.cmcmedicaldevices.com |
Bisaf Sp. z o.o. Rdestowa 5 54-530 Wrocław Polska 24h@bisaf.pl +48 503411527 www.bisaf.pl |
Bestsellery
224,75 PLN per package (25 pieces)
8,99 PLN per piece
36,89 zł za 1 opakowanie
14,99 PLN per piece
Previous
Next
Last watched
486,00 PLN per package (25 pieces)
86,90 PLN per package (10 pieces)
8,69 PLN per piece
1999,99 PLN per package (100 pieces)
Previous
Next